|
Quantity
|
Out of stock
|
||
|
|
|||
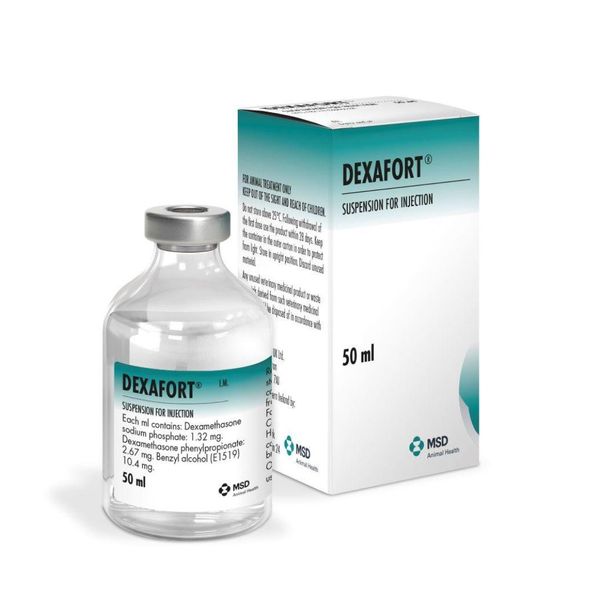
Instructions for use Dexafort 50 ml,
Intervet Intervet Dexafort is a corticosteroid for the relief of inflammation and autoimmune diseases in animals. It is used for dogs, cats and other pets. Dosage for intramuscular use Dogs: 0.5 - 1 ml. Cats: 0.25 - 0.5 ml. Composition and release form Dexafort is a hormonal drug containing 2.67 mg of dexamethasone phenylpropionate and 1.32 mg of dexamethasone sodium phosphate as active ingredients in 1 ml, as well as auxiliary components: 4.0 mg of sodium chloride, 11.4 mg of sodium citrate, 10.4 mg of benzyl alcohol, 0.4 mg of methylcellulose MX 50 and water for injection up to 1 ml. It is a sterile aqueous suspension for injection of white color.
During storage, sediment may form, which disintegrates into a homogeneous suspension when shaken. Packaged in 50 ml glass bottles, which are packed in cardboard boxes. Pharmacological properties Dexamethasone, which is part of the drug, is a synthetic analogue of cortisol - a glucocorticosteroid hormone of the adrenal cortex. It has a more pronounced glucocorticosteroid effect, has an anti-inflammatory, anti-edematous, desensitizing and anti-allergic effect. The mechanism of action of the hormone is to inhibit the release of inflammation mediators by eosinophils and mast cells. Dexamethasone affects all stages of the inflammatory process: it inhibits the synthesis of prostaglandins at the level of arachidonic acid, the synthesis of "pro-inflammatory cytokines" (interleukin 1, tumor necrosis factor alpha, etc.), and also increases the resistance of the cell membrane to the action of various damaging factors. Dexafort stimulates steroid receptors of lymphocytes, promoting the formation of lipocortins with anti-edematous activity. It inhibits the proliferation of lymphoid tissue and cellular immunity, disrupts the kinetics of T-lymphocytes, reducing their cytotoxic activity. B-lymphocytes are more resistant to glucocorticoids, but the introduction of the drug in high doses leads to a decrease in the concentration of immunoglobulins, which is associated with an initial increase in their catabolism and subsequent inhibition of synthesis. Dexafort is characterized by rapid action and duration of effect. After intramuscular administration of dexamethasone sodium phosphate begins to be rapidly absorbed from the injection site. Dexamethasone phenylpropionate is absorbed more slowly and provides a long-lasting effect. The maximum concentration of dexamethasone in blood plasma is detected after 60 minutes. The therapeutic concentration in the blood serum is maintained for 30-96 hours depending on the type of animal. Bioavailability after intramuscular administration is 100%. Dexamethasone is biotransformed in the liver and partly in fibroblasts involved in the metabolism process. Metabolites are excreted mainly with feces and urine. Indications Prescribed for cattle, horses, pigs, sheep, goats, dogs and cats for the treatment of inflammatory processes and diseases of allergic and autoimmune etiology (allergic dermatitis, eczema, post-traumatic edema, bronchial asthma, joint diseases, acute mastitis). Dosage and method of administration Dexafort is administered to cattle, horses, pigs, sheep and goats intramuscularly, to dogs and cats intramuscularly or subcutaneously once in the following doses: cattle and horses - 10 ml, calves, foals, sheep, goats and pigs - 1 - 3 ml, dogs - 0.5 - 1 ml, cats - 0.25 - 0.5 ml. If necessary, the drug is used again after 7 days.
Before each use, shake the bottle with dexafort thoroughly until a homogeneous suspension is obtained. In the treatment of inflammation complicated by pathogenic and opportunistic bacterial microflora, dexafort is prescribed only in combination with broad-spectrum antibacterial drugs. Side effects Polyuria (increased urine output), polydipsia (intense unquenchable thirst) and polyphagia (excessive feed intake) may occur. With prolonged use, Cushing's syndrome may develop, clinically manifested by intense thirst, frequent urination with urinary incontinence both day and night, extensive symmetrical alopecia, increased appetite, drowsiness, muscle weakness, pendulous abdomen, weight loss and osteoporosis. The use of corticosteroids in lactating cows may lead to a short-term decrease in milk production. The use of corticosteroids to stimulate labor may cause a decrease in fetal viability, as well as an increase in the incidence of retained placenta. Contraindications Increased individual sensitivity to the components of the drug. It is prohibited to use Dexafort in pregnant animals in the last trimester of pregnancy, as this may lead to premature birth or abortion. Dexafort should not be administered simultaneously with vaccines due to the immunosuppressive effect of corticosteroids. Do not administer to animals with viral and fungal diseases, diabetes mellitus,
osteoporosis, hyperadrenocorticism, kidney disease and heart failure. Dexafort should be administered to pregnant animals with great caution in the first and second trimesters of pregnancy. Special instructions Slaughter of cattle for meat is permitted 48 days after the last administration of the drug, horses - 24 days after the last administration of the drug. Milk from cows for 5 days after the last administration of the drug is prohibited for human consumption, such milk can be used as animal feed after heat treatment. Meat from animals forced to be slaughtered before the expiration of the specified period can be used to obtain meat and bone meal. Storage conditions With precautions according to list B. In a dry place protected from light and inaccessible to children and animals at a temperature of 15 to 25 ° C. Shelf life is 5 years. After opening the bottle, the drug should be used within 8 weeks, observing the rules of asepsis. Acute mastitis).
Dosage and method of administration Dexafort is administered to cattle, horses, pigs, sheep and goats intramuscularly, to dogs and cats intramuscularly or subcutaneously once in the following doses: cattle and horses - 10 ml, calves, foals, sheep, goats and pigs - 1 - 3 ml, dogs - 0.5 - 1 ml, cats - 0.25 - 0.5 ml. If necessary, the drug is used again after 7 days. Before each use, the bottle with dexafort is thoroughly shaken until a homogeneous suspension is obtained.
In the treatment of inflammation complicated by pathogenic and opportunistic bacterial microflora, dexafort is prescribed only in combination with broad-spectrum antibacterial drugs. Side effects Polyuria (increased urine production), polydipsia (intense unquenchable thirst) and polyphagia (excessive feed consumption) may occur. With prolonged use, Cushing's syndrome may develop, clinically manifested by intense thirst, frequent urination with urinary incontinence both day and night, extensive symmetrical alopecia, increased appetite, drowsiness, muscle weakness, pendulous abdomen, weight loss and osteoporosis. The use of corticosteroids in lactating cows may lead to a short-term decrease in milk production. The use of corticosteroids to stimulate labor may cause a decrease in fetal viability, as well as an increase in the incidence of retained placenta. Contraindications Increased individual sensitivity to the components of the drug. It is prohibited to use Dexafort in pregnant animals in the last trimester of pregnancy, as this may lead to premature birth or abortion. Dexafort should not be used simultaneously with vaccines due to the immunosuppressant effect of corticosteroids.
Should not be used in animals with viral and fungal diseases, diabetes mellitus, osteoporosis, hyperadrenocorticism, kidney disease and heart failure. Dexafort should be administered to pregnant animals with great caution in the first and second trimesters of pregnancy. Special instructions Slaughter of cattle for meat is permitted 48 days after the last administration of the drug, horses - 24 days after the last administration of the drug. Milk from cows for 5 days after the last administration of the drug is prohibited for human consumption, such milk can be used as animal feed after heat treatment. Meat from animals forced to be killed before the expiration of the specified period can be used to obtain meat and bone meal.
Storage conditions With precautions according to list B. In a dry place protected from light and inaccessible to children and animals at a temperature of 15 to 25 ° C. Shelf life is 5 years. After opening the bottle, the drug should be used within 8 weeks, observing the rules of asepsis. Acute mastitis). Dosage and method of administration Dexafort is administered to cattle, horses, pigs, sheep and goats intramuscularly, to dogs and cats intramuscularly or subcutaneously once in the following doses: cattle and horses - 10 ml, calves, foals, sheep, goats and pigs - 1 - 3 ml, dogs - 0.5 - 1 ml, cats - 0.25 - 0.5 ml. If necessary, the drug is used again after 7 days. Before each use, the bottle with dexafort is thoroughly shaken until a homogeneous suspension is obtained. In the treatment of inflammations complicated by pathogenic and opportunistic bacterial microflora, dexafort is prescribed only in combination with broad-spectrum antibacterial drugs. Side effects Polyuria (increased urine production), polydipsia (intense unquenchable thirst) and polyphagia (excessive feed intake) may occur. With prolonged use, Cushing's syndrome may develop, clinically manifested by intense thirst, frequent urination with urinary incontinence both day and night, extensive symmetrical alopecia, increased appetite, drowsiness, muscle weakness, pendulous abdomen, weight loss and osteoporosis. Use of corticosteroids in dairy cows
in may lead to a short-term decrease in milk production. The use of corticosteroids to stimulate labor may cause a decrease in fetal viability, as well as an increase in the frequency of retained placenta.
Contraindications
Hypersensitivity to the components of the drug.
Dexafort should not be used in pregnant animals in the last trimester of pregnancy, as this may lead to premature birth or abortion.
Dexafort should not be used simultaneously with vaccines due to the immunosuppressive effect of corticosteroids. It should not be used in animals with viral and fungal diseases, diabetes mellitus, osteoporosis, hyperadrenocorticism, kidney disease and heart failure. Dexafort should be administered with great caution to pregnant animals in the first and second trimesters of pregnancy.
Special instructions
Slaughter of cattle for meat is allowed 48 days after the last administration of the drug, horses - 24 days after the last administration of the drug. Milk from cows is prohibited for human consumption within 5 days after the last administration of the drug, such milk can be used as animal feed after heat treatment. Meat of animals forced to be killed before the expiration of the specified period can be used to obtain meat and bone meal. Storage conditions With precautions according to list B. In a dry place protected from light and inaccessible to children and animals at a temperature of 15 to 25 ° C. Shelf life is 5 years. After opening the bottle, the drug should be used within 8 weeks, observing the rules of asepsis. cats - 0.25 - 0.5 ml. If necessary, the drug is re-used after 7 days. Before each use, the bottle with dexafort is thoroughly shaken until a homogeneous suspension is obtained. In the treatment of inflammations complicated by pathogenic and opportunistic bacterial microflora, dexafort is prescribed only in combination with broad-spectrum antibacterial drugs. Side effects Polyuria (increased urine production), polydipsia (intense unquenchable thirst) and polyphagia (excessive feed consumption) may occur. With prolonged use, Cushing's syndrome may develop, clinically manifested by intense thirst, frequent urination with urinary incontinence both day and night, extensive symmetrical alopecia, increased appetite, drowsiness, muscle weakness, pendulous abdomen, weight loss and osteoporosis. The use of corticosteroids in lactating cows may lead to a short-term decrease in milk production. The use of corticosteroids to stimulate labor may cause a decrease in fetal viability, as well as an increase in the incidence of retained placenta. Contraindications Increased individual sensitivity to the components of the drug. It is prohibited to use Dexafort in pregnant animals in the last trimester of pregnancy, as this may lead to premature birth or abortion. Dexafort should not be administered simultaneously with vaccines due to the immunosuppressant effect of corticosteroids. Should not be administered to animals with viral and fungal diseases, diabetes mellitus, osteoporosis, hyperadrenocorticism, kidney disease and heart failure. Dexafort should be administered with great caution to pregnant animals in the first and second trimesters of pregnancy. Special instructions Slaughter of cattle for meat is permitted 48 days after the last administration of the drug, horses - 24 days after the last administration of the drug. Milk from cows for 5 days after the last administration of the drug is prohibited for human consumption, such milk can be used as animal feed after heat treatment. Meat of animals forced to be slaughtered before the expiration of the specified period can be used to produce meat and bone meal. Storage conditions With precautions according to list B. In a dry place, protected from light and inaccessible to children and animals, at a temperature of 15 to 25 ° C. Shelf life is 5 years. After opening the bottle, the drug should be used within 8 weeks, observing the rules of asepsis. Cats - 0.25 - 0.5 ml. If necessary, the drug is used again after 7 days. Before each use, the bottle with dexafort is thoroughly shaken until a homogeneous suspension is obtained. In the treatment of inflammation complicated by pathogenic and opportunistic bacterial microflora, dexafort is prescribed only in combination with broad-spectrum antibacterial drugs. Side effects Polyuria (increased urine production), polydipsia (strong unquenchable thirst) and polyphagia (excessive food consumption) are possible. With prolonged use, Cushing's syndrome may develop, clinically manifested by severe thirst, frequent urination with urinary incontinence both day and night, extensive symmetrical alopecia, increased appetite, drowsiness, muscle weakness, pendulous belly, weight loss and osteoporosis. Use of corticosteroids
in lactating cows may result in a short-term decrease in milk production. The use of corticosteroids to stimulate labor may cause a decrease in fetal viability and an increase in the incidence of retained placenta. Contraindications Hypersensitivity to any of the components of the drug. It is prohibited to administer Dexafort to pregnant animals in the last trimester of pregnancy, as this may lead to premature birth or abortion. Dexafort should not be administered simultaneously with vaccines due to the immunosuppressant effect of corticosteroids. It should not be administered to Animals with viral and fungal diseases, diabetes mellitus, osteoporosis, hyperadrenocorticism, kidney disease and heart failure. Dexafort should be administered to pregnant animals in the first and second trimesters of pregnancy with great caution. Special instructions Slaughter of cattle for meat is permitted 48 days after the last administration of the drug, horses - 24 days after the last administration of the drug. Milk from cows within 5 days after the last administration of the drug is prohibited for human consumption, such milk can be used as animal feed after heat treatment. Meat of animals forced to be killed before the expiration of the specified period can be used to produce meat and bone meal. Storage conditions With precautions according to list B. In a dry place protected from light and inaccessible to children and animals at a temperature of 15 to 25 ° C.
Shelf life is 5 years.
After opening the bottle, the drug should be used within 8 weeks, observing the rules of asepsis. frequent urination with urinary incontinence both day and night, extensive symmetrical alopecia, increased appetite, drowsiness, muscle weakness, pendulous abdomen, weight loss and osteoporosis. The use of corticosteroids in dairy cows can lead to a short-term decrease in milk production. The use of corticosteroids to stimulate labor can cause a decrease in fetal viability, as well as an increase in the incidence of retained placenta.