|
Quantity
|
Out of stock
|
||
|
|
|||
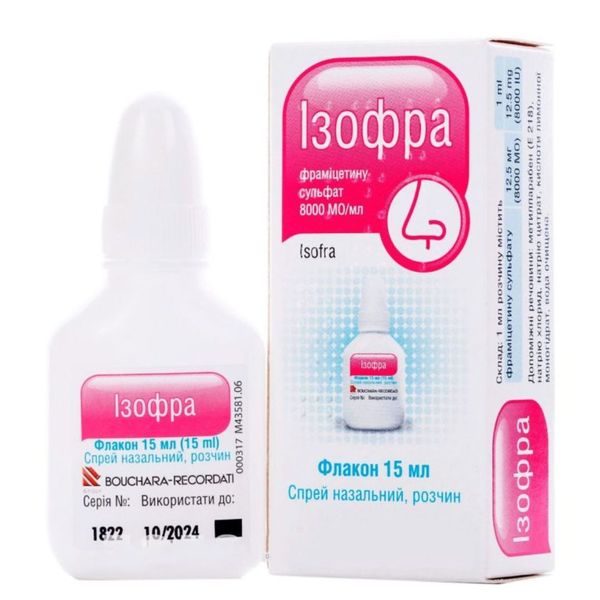
Composition
active ingredient: framycetin;
1 ml framycetin sulfate 12.5 mg (8000 IU);
excipients: methylparaben (E 218), sodium chloride, sodium citrate, citric acid monohydrate, purified water.
Dosage form
Nasal spray, solution.
Main physicochemical properties: transparent liquid.
Pharmacological group
De-swelling and other drugs for topical use in case of diseases of the nasal cavity. ATX code
R01A X08.
Pharmacological properties
Pharmacodynamics.
Framycetin is an antibiotic from the aminoglycoside group for topical use. The concentration of framycetin, which is achieved with local application, provides its bactericidal activity against gram-positive and gram-negative microorganisms that cause the development of infectious processes in the upper respiratory tract.
Framycetin is active against Corynebacterium, Listeria monocytogenes, Staphylococcus meti-S, Acinetobacter (mainly Acinetobacter baumanii), Branhamella catarrhalis, Campylobacter, Citrobacter freundii, Citrobacter koseri, Enterobacter aerogenes, Enterobacter cloacae, Escherichia coli, Haemophilus influenza, Klebsiella, Morganella morganii, Proteus mirabilis, Proteus vulgaris, Providencia rettgeri, Salmonella, Serretia, Shigella, Yersinia.
The Pasteurella species is moderately sensitive to the action of framycetin.
Bacteria resistant to the action of framycetin are Enterococci, Nocardia asteroides, Staphylococcus meti-R* (resistance is about 30-50%, more pronounced hospital), Streptococcus, Alcaligenes denitrificans, Burkholderia, Flavobacterium sp., Providencia stuartii, Pseudomonas aeruginosa, Stenotrophomonas maltophilia, resistant anaerobic bacteria, Chlamydia, Mycoplasma, Rickettsia.
Pharmacokinetics.
Pharmacokinetics studies of the drug have not been conducted due to low systemic absorption.
Indications
As part of combination therapy for infectious and inflammatory diseases of the upper respiratory tract, incl. rhinitis, nasopharyngitis, sinusitis in the absence of damage to the walls of the nasal sinus, prevention and treatment of inflammatory processes after surgery.
Contraindications
Hypersensitivity (allergy) to any of the components of the drug, including framycetin and other antibiotics of the aminoglycoside group.
Age up to 1 year.
Interaction with other drugs and other types of interaction
Unknown.
In case of using any other drugs for local use, you should definitely inform your doctor.
Special instructions for use
The drug should not be used to rinse the paranasal sinuses. With prolonged use of the drug, resistant strains of microorganisms may develop. If there is no therapeutic effect within 7 days of treatment, the drug should be discontinued.
Use during pregnancy or breastfeeding.
The effectiveness and safety of using the drug during pregnancy have not been sufficiently studied.
A toxic effect on the cochleovestibular apparatus of the fetus is possible. Systemic penetration through the mucous membrane is possible, so it should not be prescribed to this category of patients.
Use of the drug during breastfeeding is not recommended, since aminoglycosides penetrate into breast milk.
Ability to affect the reaction rate when driving vehicles or other mechanisms.
No cases of influence on the reaction rate when driving vehicles or working with other mechanisms have been identified.
Method of administration and dosage
Adults are prescribed 1 injection in each nasal passage 4-6 times a day.
Children from 1 year of age are prescribed 1 injection in each nasal passage 3 times a day. Inject by pressing the bottle. When using, hold the bottle vertically and tilt your head slightly forward so that the solution injected into the nasal passage is in the form of a spray, and not a stream of liquid.
The duration of treatment is up to 10 days.
Children.
Use as prescribed by a doctor for the treatment of children over 1 year old.
Overdose
No cases of overdose have been reported.
Side effects
Spontaneous reports of the following adverse reactions with the specified frequency in patients treated with framycetin during the post-marketing period and in the scientific literature (very common (≥ 1/10), common (≥ 1/100, <1/10); uncommon (≥ 1 / 1000, <1/100); isolated (1/10000, <1/1000); rare (less than <1/10000)).
Rare: allergic skin reactions (urticaria, itching).
Shelf life
3 years.
Storage conditions
Keep out of reach of children at a temperature not exceeding 25 ° C.
Packaging
15 ml in a spray bottle in a cardboard box.
Category of dispensing
On prescription.