|
Quantity
|
Out of stock
|
||
|
|
|||
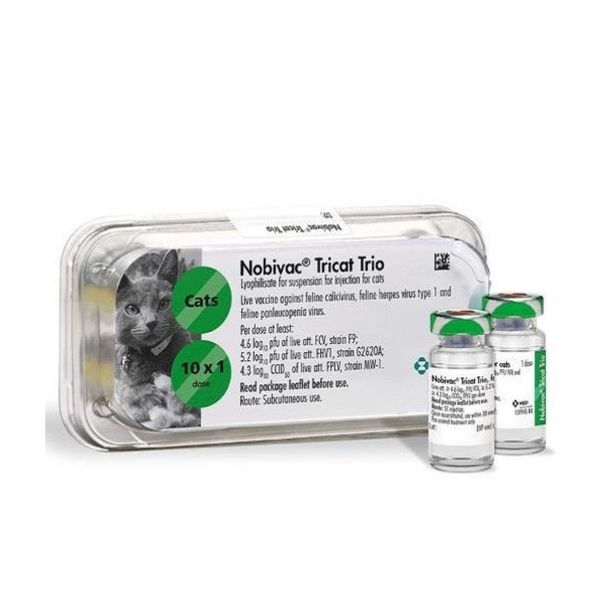
Vaccine against calicivirus, viral rhinotracheitis and panleukopenia of cats live dry with solvent
Composition
One dose of the vaccine contains active substances, live attenuated viruses:
- feline calicivirus (strain F9), not less than 4.6 log10 PFU;
- feline rhinotracheitis virus (strain G2620A), not less than 5.2 log10 PFU;
- feline panleukopenia virus (strain MW-1), not less than 4.3 log10 TCID50;
and excipients: hydrolyzed gelatin, sorbitol, pancreatic hydrolysate of casein, disodium hydrogen phosphate dihydrate, water for injection.
The solvent is a sterile phosphate buffer solution, which includes disodium hydrogen phosphate dihydrate, potassium dihydrogen phosphate, water for injection.
Indications for use
For active immunization of cats to reduce virus shedding and clinical manifestations caused by calicivirus and/or feline rhinotracheitis virus, as well as to prevent clinical manifestations, virus shedding and leukopenia caused by feline panleukopenia virus.
Dosage form
Lyophilisate for preparation of injection suspension (vaccine) and injection solution (solvent).
Method of administration
For subcutaneous administration
Dosage form
The vaccine is packaged in 1 dose (volume before lyophilization - 0.5 cm3), solvent - 1 ml in glass vials. Vials with vaccine and solvent are hermetically sealed with halogenated butyl rubber stoppers, reinforced with aluminum caps, and packed in 5 or 25 pieces in cardboard or plastic boxes with instructions for use in Russian included.
Shelf life
The shelf life of the vaccine is 33 months, and the solvent is 60 months from the date of manufacture.
Storage conditions
Store in a place protected from light at a temperature of 2 to 8 °C. It is permissible to store the solvent separately from the vaccine at a temperature of 15 to 25 °C.