|
Quantity
|
Out of stock
|
||
|
|
|||
International nonproprietary name: torasemide.
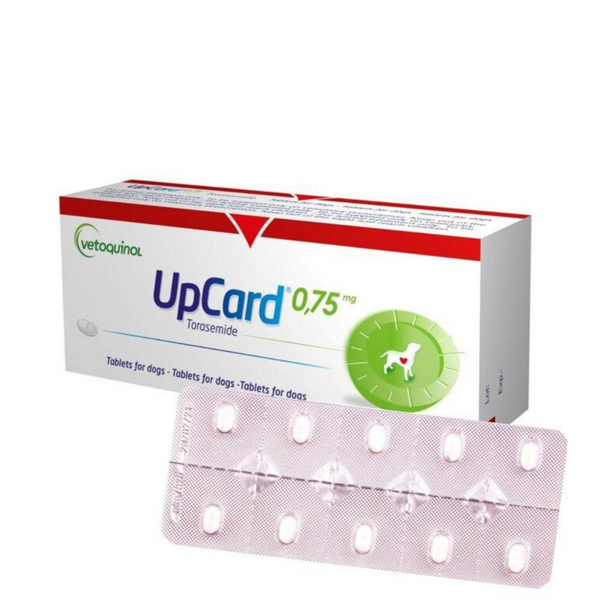
Dosage form: tablets for oral use.
In appearance, ApKard is a white to cream-colored, oblong tablet with dividing notches (divisible).
Composition
ApKard contains torasemide as an active substance.
The drug is available in three modifications: with a torasemide content of 0.75 mg, 3 and 7.5 mg. As auxiliary substances, ApKard contains lactose monohydrate, povidone, crospovidone, sodium lauryl sulfate, microcrystalline cellulose, sodium stearyl fumarate and bacon flavoring.
Pharmacological properties
ApKard belongs to the group of drugs of the loop diuretics.
The mechanism of action of torasemide is to inhibit the reabsorption of sodium and chloride ions in the lumen of the tubules of the medullary part of the ascending limb of the loop of Henle, which leads to their increased excretion and a decrease in the osmolarity of the interstitial space in the medullary layer of the kidney, which in turn reduces the reabsorption of free water and leads to an increase in its excretion from the body.
After oral administration of the drug, torasemide is rapidly absorbed, enters the bloodstream, reaching a maximum concentration in less than 1 hour. Bioavailability is 90%.
Torasemide is slightly metabolized (about 7-10%) in the liver and excreted from the body unchanged, mainly through the urinary system. The half-life is 7 hours.
According to the degree of impact on the body, ApCard belongs to low-hazard substances (hazard class 4 according to GOST 12.1.007-76).
Directions for use
ApCard is used in dogs as a diuretic to relieve clinical signs associated with congestive heart failure, including edema and effusions.
ApCard is administered individually, orally, regardless of feeding regimen, at a dose of 0.1-0.6 mg torasemide per 1 kg of animal weight once daily.
In most dogs, the condition can be stabilized with a torasemide dose of less than or equal to 0.3 mg/kg of animal weight once daily.
The torasemide dose should be titrated to an acceptable level for the patient, taking into account renal function and electrolyte status. If it is necessary to change the diuretic level, the dose can be increased or decreased within the recommended dose range in increments of 1 U mg/kg of animal weight. Dose adjustments should be made as prescribed by a veterinarian.
Renal function, hydration level, and electrolyte balance in the blood should be monitored:
at the start of therapy;
24-48 hours after the start of therapy;
24-48 hours after changing the dose;
if adverse effects develop.
If long-term therapy is necessary, continue treatment with the minimum effective dose.
Contraindications
ApCard is contraindicated for use in animals with renal failure, severe dehydration, hypovolemia or hypotension, as well as in case of increased individual sensitivity to the drug or its components.
It is prohibited to use ApCard simultaneously with other loop diuretics. ApCard should be prescribed with caution when using antihypertensive drugs.
ApCard is not intended for use in productive animals.
Special features of use
ApCard should be used with caution in diabetes mellitus and in dogs that have previously been prescribed high doses of another loop diuretic.
ApCard is not intended for use in animals during pregnancy and lactation, as well as in breeding animals.
You should avoid skipping the next dose of the drug because this may lead to a decrease in its therapeutic effectiveness. If one or more doses of the drug are missed, the course of treatment should be resumed in the prescribed dosages and application schedule. Do not administer a double dose to compensate for the missed dose.
Overdose
In case of drug overdose, animals may experience dehydration, electrolyte imbalance, renal failure, anorexia, weight loss and cardiovascular collapse. Symptomatic treatment is performed.
Peculiarities of action
Peculiarities of action during the first use of ApKard and its cancellation have not been established.
Side effects
When using ApKard, the following side effects may be observed: increased renal parameters (creatinine and urea nitrogen) and renal failure, hemoconcentration, polyuria and/or polydipsia, electrolyte deficiency and dehydration, vomiting, diarrhea, decreased volume or absence of feces, soft texture of feces.
Depending on the severity of adverse reactions, dose adjustment, symptomatic treatment or discontinuation of the drug is possible. The decision is made by the attending veterinarian after assessing the clinical condition of the animal and the benefit/risk ratio.
If the animal develops allergic reactions, the use of the drug is discontinued and symptomatic treatment is prescribed.
Personal preventive measures
Pr
and when working with the drug, you should follow the general safety rules provided for when working with medicinal products for veterinary use.
When working, you should follow the general rules of personal hygiene.
Empty packages from the drug are prohibited from being used for household purposes, they are subject to disposal with household waste.
People with hypersensitivity to the components of the drug should avoid direct contact with the drug ApKard.
In case of accidental contact of the drug with the skin or mucous membranes, immediately rinse them with plenty of water. If allergic reactions occur or if the drug accidentally enters the human body, you should immediately contact a medical institution (have instructions for use or a label with you).
Release form
ApKard is available in tablets, packaged in 10 pieces in foil blisters. Blisters with the drug are packed in cardboard boxes of 3 blisters (30 tablets) or 10 blisters (100 tablets). Each consumer package is supplied with instructions for use.
Storage
ApCard is stored in the manufacturer's sealed packaging, separately from food and feed, in a place protected from direct sunlight at a temperature of 5 to 25 °C. ApCard should be stored out of the reach of children.
Shelf life
The shelf life of the drug, subject to storage conditions, is 3 years from the date of manufacture. The unused portion of the tablet may be stored in a blister for no more than 7 days.
It is prohibited to use ApCard after the expiration date.
The unused drug is disposed of in accordance with legal requirements.
Dispensed without a prescription from a veterinarian.