|
Quantity
|
Out of stock
|
||
|
|
|||
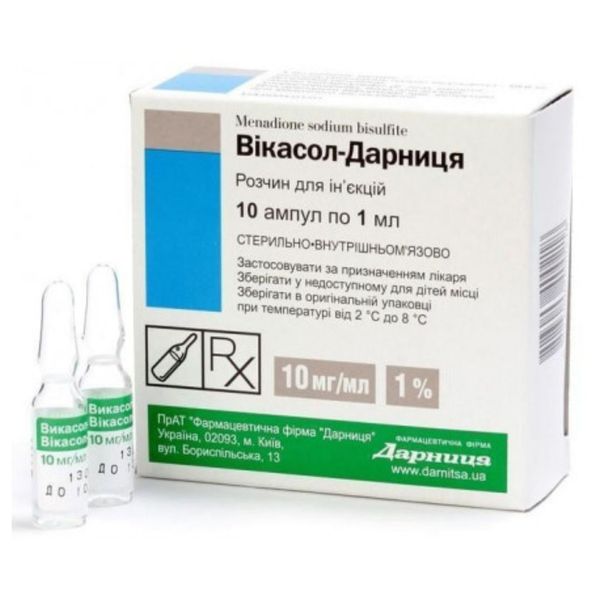
Vikasol-Darnitsa instructions for use
Storage
active substance: menadione sodium bisulfite;
1 ml of solution contains vikasol (menadione sodium bisulfite) 10 mg;
excipients: sodium metabisulfite (E 223), hydrochloric acid, water for injections.
Medicinal form
Solution for injections.
Main physicochemical properties: transparent colorless or slightly yellowish liquid.
Pharmacotherapeutic group
Vitamin K and other hemostatic agents. ATX code B02B A02.
Pharmacological properties
Pharmacodynamics.
Vitamin K exists in several forms. The main form of vitamin K - vitamin K1 (phylloquinone) is found in plants, especially green leafy vegetables. Another form of vitamin K is vitamin K2 (menaquinone), which can be absorbed in limited amounts. It is produced by bacteria in the lower part of the small intestine and in the large intestine.
Vikasol-Darnytsia is a synthetic analogue of water-soluble vitamin K and is considered as vitamin K3 (menadione), promotes the synthesis of prothrombin and proconvertin, increases blood coagulation by increasing the synthesis of II, VII, IX, X coagulation factors. It has a hemostatic effect (with vitamin K deficiency, increased bleeding occurs). In the blood, prothrombin (factor II) in the presence of thromboplastin and Ca2+, with the participation of proconvertin (factor VII), factors IX (Christmas factor), X (Stewart-Prawer factor) turns into thrombin, under the influence of which fibrinogen turns into fibrin, which is the basis of a blood clot (thrombus). Vikasol-Darnytsia as a substrate stimulates K-vitamin reductase, which activates vitamin K and ensures its participation in the hepatic synthesis of K-vitamin-dependent plasma factors of hemostasis.
The role of vitamin K in the human and animal body is not limited to the effect on the biosynthesis of procoagulants. It affects bioenergetics and a number of anabolism processes. It is important for the exchange of macroergic compounds, for the formation of ATP. K-vitamin deficiency leads to a deficiency of ATP and a decrease in energy supply for the biosynthesis of many compounds. The latter include not only procoagulants, but also other rapidly resynthesized proteins, including a number of enzymes not directly related to the blood coagulation process. With a deficiency of vitamin K, the biosynthesis of some biologically active substances of a non-protein nature is also weakened: serotonin, histamine, acetylcholine. The onset of effect is 8-24 hours after intramuscular administration.
Pharmacokinetics.
After intramuscular administration, it is easily and quickly absorbed into the systemic bloodstream. Binding to plasma proteins is reversible. The blood of patients with very low albumin content binds vitamin K weakly or does not bind it at all. It accumulates mainly in the liver, spleen, and myocardium. The transformation process takes place most intensively in the myocardium and skeletal muscles, and somewhat more slowly in the kidneys. It is excreted by the kidneys and bile only exclusively in the form of metabolites. Metabolites of vitamin K (monosulfate, phosphate and diglucuronide-2-methyl-1,4-naphthoquinone) are excreted in the urine - up to 70%. High concentrations of vitamin K in feces are due to the fact that it is synthesized by intestinal microflora.
Clinical characteristics.
Indication
Vikasol is prescribed for bleeding and against the background of hypoprothrombinemia caused by jaundice, acute hepatitis, capillary and parenchymal bleeding. After surgical interventions and injuries, with bleeding from peptic ulcer disease of the stomach and duodenum, pronounced symptoms of acute radiation sickness, long-term nasal and hemorrhoidal bleeding.
The drug should be prescribed for hemorrhagic phenomena in premature children, uterine pre-climacteric and juvenile bleeding, spontaneous bleeding, preparation for surgical interventions, if there is a risk of bleeding in the postoperative period, pulmonary bleeding, hemorrhagic phenomena against the background of septic diseases.
In addition, indications for the appointment of Vikasol-Darnytsia are bleeding and hypoprothrombinemia caused by an overdose of phenylin, neodicumarin, other anticoagulants - vitamin K antagonists.
Contraindications of Vikasol-Darnytsia
Hypersensitivity to the components of the medicinal product. Hypercoagulation, thromboembolism. Hemolytic disease of newborns. Severe liver failure. Deficiency of glucose-6-phosphate dehydrogenase.
III trimester of pregnancy (prophylactic appointment of vitamin K is ineffective due to its low permeability through the placenta).
Interaction with other medicinal products and other types of interactions.
With simultaneous use with oral anticoagulants, a decrease in the anticoagulant effect is possible. Weakens the effect of indirect anticoagulants (including coumarin and indandione derivatives). It does not affect the anticoagulant activity of heparin.
Simultaneous use with broad-spectrum antibiotics, quinidine, quinine, salicylates in high doses, sulfonamides requires an increase in the dose of vitamin K.
With simultaneous use with hemolytic drugs, the risk of side effects increases.
When used simultaneously with aggregants and fibrinolysis inhibitors, their hemostatic effect is potentiated.
Features of the use of the drug
It should be taken into account that inhibition of platelet aggregation may occur when using Vikasol. In hemophilia, thrombocytopenic purpura and Werlhoff's disease, the drug is ineffective.
For the prevention of hemorrhagic disease of newborns, it is better to use phytomenadione than menadione sodium bisulfite, because it less often causes hyperbilirubinemia and hemolytic anemia in newborns (including premature babies).
Use during pregnancy or breastfeeding.
Vikasol-Darnytsia belongs to the drugs of risk during pregnancy. The use of the drug during pregnancy in the Ι and ΙΙ trimesters is possible according to indications, if the benefit to the mother exceeds the degree of risk to the fetus. The prophylactic appointment of vitamin K in the III trimester of pregnancy is ineffective due to its low permeability through the placenta.
If it is necessary to prescribe a medicinal product, breastfeeding should be stopped.
The ability to influence the speed of reaction when driving vehicles or other mechanisms.
The drug does not affect the speed of reaction when driving a motor vehicle or working with other mechanisms. If dizziness is observed during treatment with the drug, you should refrain from driving vehicles and working with mechanisms.
Method of application and dosage of Vikasol-Darnytsia
Administer the drug intramuscularly.
For adults, a single dose is 10 mg, the maximum single dose is 15 mg; the maximum daily dose is 30 mg.
The duration of treatment is 3-4 days, after a 4-day break, the course can be repeated if necessary.
For surgical interventions with possible severe parenchymal bleeding, prescribe within 2-3 days before surgery.
Children: up to 1 year – 2-5 mg/day, 1-2 years – 6 mg/day, 3-4 years – 8 mg/day, 5-9 years – 10 mg/day, 10-18 years – 15 mg /day Divide the dose into 2 administrations. The duration of treatment for children is determined by the doctor.
Children.
The drug should be used in pediatric practice.
Overdose
Symptoms: hypervitaminosis of vitamin K, manifested by hyperprothrombinemia and hyperthrombinemia, hyperbilirubinemia, jaundice, increased activity of liver enzymes; abdominal pain, constipation or diarrhea, general excitement, agitation and skin rash were noted. In isolated cases, children develop toxicosis, manifested by convulsions.
Treatment. Cancel the medicine. Anticoagulants should be prescribed under the control of indicators of the blood coagulation system. The therapy is symptomatic.
Side effects of Vikasol-Darnytsia
From the side of the liver and biliary tract: hyperbilirubinemia, jaundice (including nuclear jaundice in infants).
From the side of the cardiovascular system: a transient decrease in blood pressure, tachycardia, weak pulse filling.
From the blood and lymphatic system: hemolytic anemia, hemolysis in newborns with congenital deficiency of glucose-6-phosphate dehydrogenase or in patients with vitamin E deficiency, thromboembolism.
From the side of the immune system: hypersensitivity reactions, including facial hyperemia, skin rash (including erythematous, urticaria), skin itching, bronchospasm.
General disorders and reactions at the injection site: dizziness, profuse sweat, change in taste, feeling of heat, pain and swelling at the injection site, discoloration of the skin in the form of spots with repeated injections in the same place. Vikasol can cause local scleroderma.
Expiration date
2 years.
Storage conditions
Store in the original packaging at a temperature of 2 °C to 8 °C. Keep out of the reach of children.
Incompatibility.
Vikasol is incompatible with sulfonamide drugs, PASK, salicylates, oxycoumarin. Incompatible in one syringe with solutions of alkalis and acids.
The active substance of the medicine quickly disintegrates under the influence of direct sunlight.
Packaging
1 ml in an ampoule; 5 ampoules each in a contoured blister pack; 2 contour envelopes in a pack.
Pharmacy dispensing category
By prescription.